
PRODUCT SPECIFICATION
Product specifications
| Product Name10E | 10E | 17E | 30E | 40E | |
| Gross ηel in % | >52 | ||||
| Physical Properties | Length* in mm | 130 | |||
| Breadth* in mm | 150 | ||||
| Height* in mm | 40 | 63 | 110 | 145 | |
| Weight in kg | 3.7 | 6.1 | 10.3 | 13.6 | |
*The values can vary with precision of +/- 2 mm
SOFC
Solid oxide fuel cells are a type of fuel cells which uses a solid oxide electrolyte to conduct negative oxygen ions from the cathode to the anode. The electrochemical oxidation of the oxygen ions with hydrogen or carbon monoxide thus occurs on the anode side. They operate at very high temperatures, typically between 500 and 1,000 °C. At these temperatures, SOFCs are not vulnerable to carbon monoxide poisoning and tolerant to intake Sulfur to a limited level. Because of these high temperatures, light hydrocarbon fuels, such as methane, propane, and butane can be internally reformed within the anode.
SOFCs have a wide variety of applications like APU, CHP with outputs from Watts to MW.
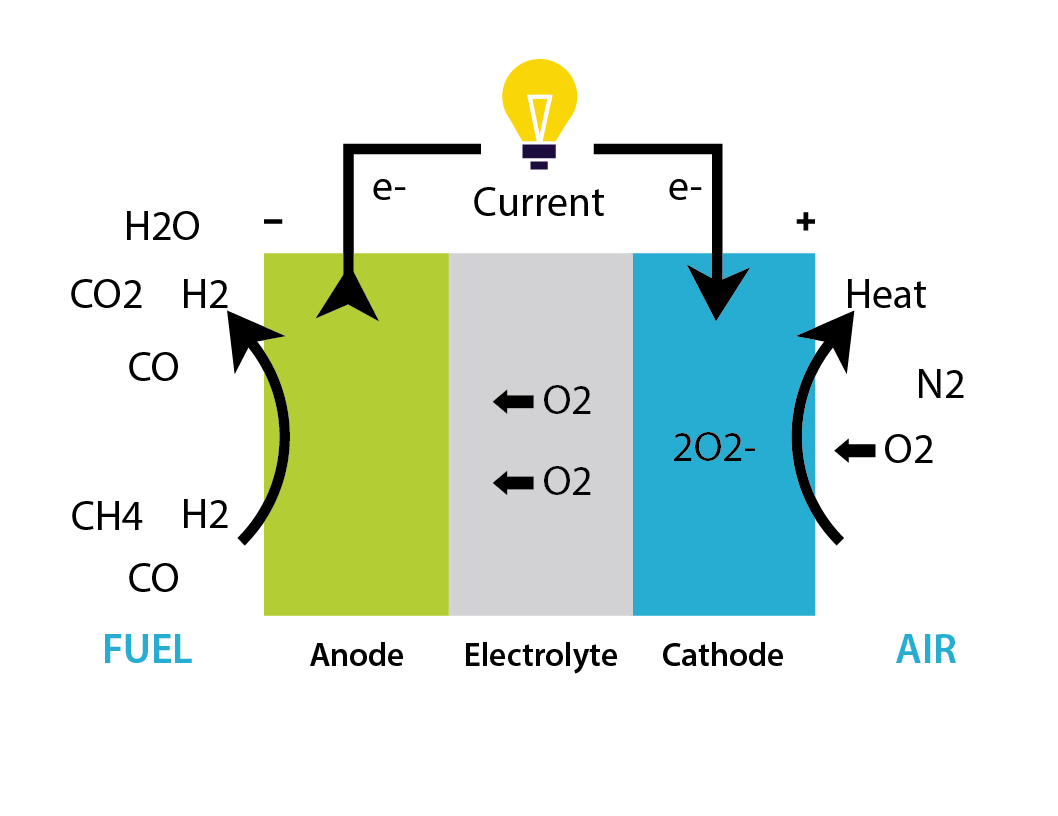
Anode – Fuel Electrode: H2 + O2- →H2O + 2e-
Cathode – Air Electrode: 1/2 O2 + 2e- → O2-
SOEC
Solid Oxide Electrolyser Cell (SOEC) is the reverse mode of SOFC operation. During this the SOEC stack produces pure hydrogen through water electrolysis by taking in the electricity and heat. The production of pure hydrogen is compelling because it is a clean fuel that can be stored easily, thus making it a potential alternative to batteries. Electrolysis is currently the most promising method of hydrogen production from water due to high efficiency of conversion and relatively low required energy input when compared to other methods. The operating conditions are similar to SOFC.
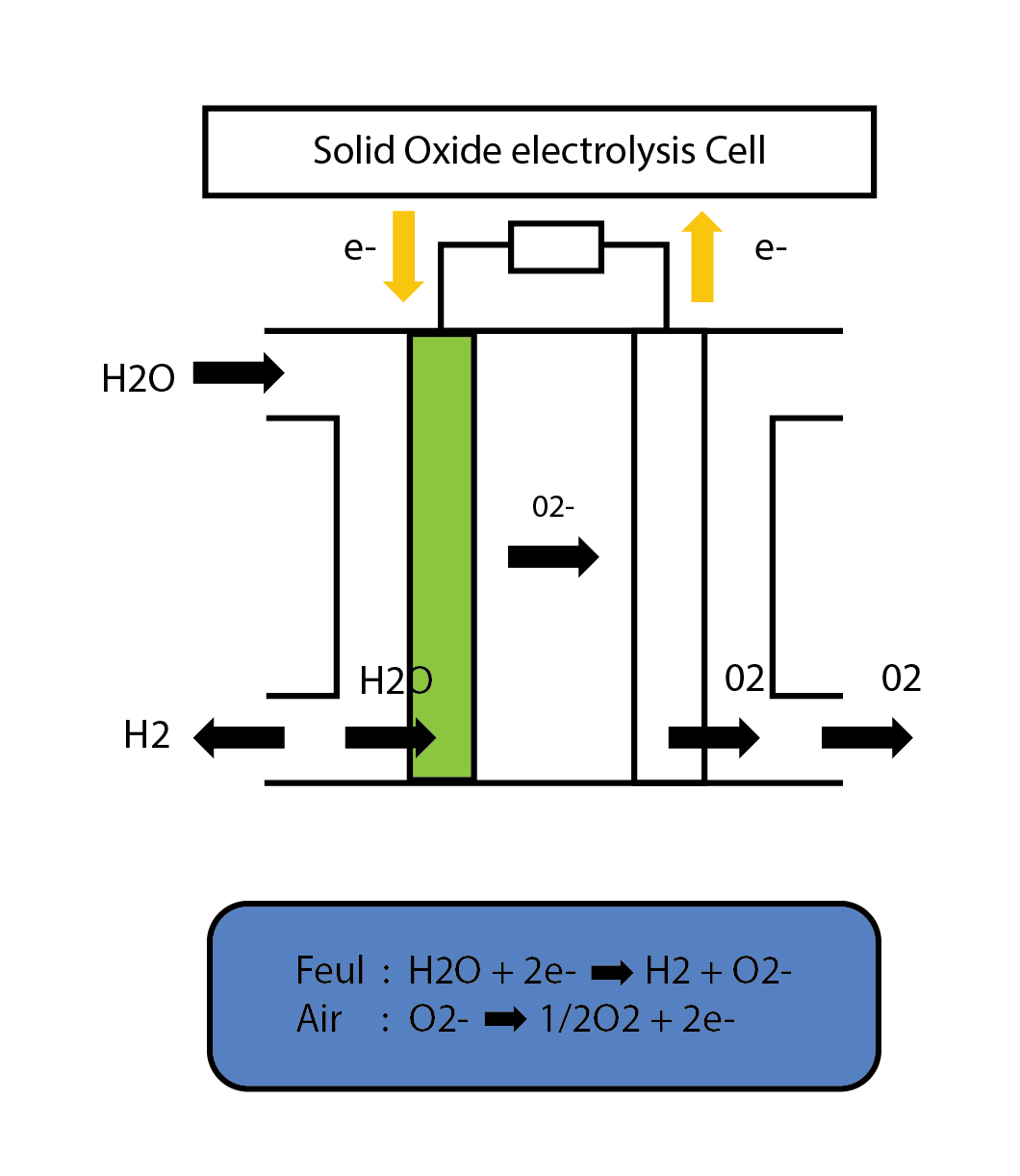
Anode – Fuel Electrode 1/2 H2O + 2e- → H2 + O2-
Cathode – Air Electrode: O2- →1/2 O2 + 2e-
rSOC
The Reversible Solid Oxide Cell (rSOC) is the combination of SOFC & SOEC operations using the same stack and can be used to produce high speciality chemicals.
